Which Equation Describes a Redox Reaction
Click again to see term. No not every chemical reaction is a redox reaction.

Half Reaction Easy Science Redox Reactions 10th Grade Science Easy Science
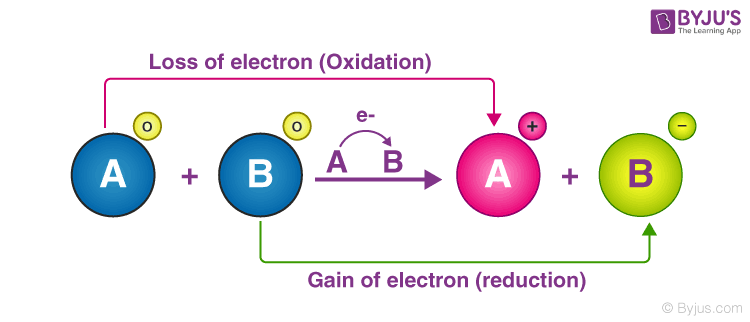
The oxidation half-reaction and the reduction half-reaction occur simultaneously.

. 2 Al s 3H 2 SO 4 aq Al 2 SO 4 3 aq 3H 2 g Reaction in which both oxidation and reduction occur is called as redox reaction. Co aq HSO4 aq HCO3 aq SO aq 12. Tap card to see definition.
Marzo 31 2022 por. HNO 3 NaOH H 2 O NaNO 3. Because there is a change in oxidation number we can confidently say that the above equation represents a redox reaction.
Cl2 2e 2Cl C. Describe the redox reaction. 3NO2g H2Ol 2HNO3aq NOg B.
2KBr aq Pb NO 3 2 aq 2KNO 3 aq PbBr 2 s H aq OH aq H 2 O l CO 32 aq HSO 4 aq HCO 3 aq SO 42 aq CaBr 2 aq H 2 SO 4 aq CaSO 4 s 2HBr aq Expert Answer. Now electrons are equal so equation works - 2Na F2 2e- -- 2Na 2F- but cancel electrons. Na -- Na e- F2 2e- -- 2F-.
3 Which one of these reactions is not an oxidation-reduction reaction. 6 CO 2 6 H 2 O light energy C 6 H 12 O 6 6 O 2. The correct option is D.
It seems that you have mixed up the oxidation and reduction half-equations. Fe2O3 3CO 2Fe 3CO2 2ZnO C 2Zn CO2 Fe2O3 2Al Al 2O3 2Fe 2CO 2NO 2CO2 N2 Which oxide is. Experts are tested by Chegg as specialists in their subject area.
Mg Mg2 2e D. 2LiOHaq H2SO4aq Li2SO4aq 2H2Ol C. CaBr2 aq H2SO4 aq CaSO4 s 2HBr g D.
Twice as many electrons in F reaction so multiply everything in Na reaction by 2 2Na -- 2Na 2e-. Which equation describes a redox reaction. H2SO4 aq 2NaOH aq 2H2O l Na2SO4 aq 11L.
Which equation represents the half-reaction that takes place at the fork. 2KBr aq Pb NO32 aq 2NO3 aq PbBrz s C. Who are the experts.
Oxidation is losing electrons while reduction is gaining electrons. What is the coefficient of silver in the final balanced equation for this reaction. Biological energy is frequently stored and released by means of redox reactions.
What is the percent by weight of acetic acid in the vinegar. Consider the redox reaction below. Vinegar is a solution of acetic acid HC2H3O2 dissolved in water.
H aq OH aq H2O 1 E. Na 2 SO 4 BaCl 2 2NaCl BaSO 4. 2HCl CaCO 3 H 2 O CaCl 2 CO 2.
6 In what order are redox reactions. In reaction A hydrogen is being reduced by gain of 1 electron in going from H2SO4 to H2. Zn s 2HCl aq -- ZnCl2 aq H2 g Which half reaction correctly describes the oxidation that is taking place.
5 Which identifies an oxidation-reduction reaction. The reaction that takes place in a chemical cell is best classi ed as A. A 554-g sample of vinegar was neutralized by 3010 mL of 0100 M NaOH.
2KNO3s 2KNO2s O2g. 2KI PbNO3 2 2KNO 3 PbI 2. Click card to see definition.
2Nas 2H2Ol 2NaOHaq H2g D. It is known as the reaction in which the exchange of electrons takes place. Photosynthesis involves the reduction of carbon dioxide into sugars and the oxidation of water into molecular oxygen.
1 Which statement best describes what is taking place in this half reaction Fe. Photosynthesis is essentially the reverse of the redox reaction in cell respiration. Some decomposition reactions are also non-redox reactions.
The oxidation reaction is defined as the reaction in which a chemical species loses electrons in a chemical reactionIt occurs when the oxidation. The equations below all show redox reactions. Which statement correctly describes a redox reaction.
The information below describes a redox reaction. Which equation describes a redox reaction. Redox reaction is defined as the reaction in which oxidation and reduction take place simultaneously.
In reaction A aluminium is being oxidized by loss of 3 electrons in going from Al s to Al2 SO43 aq. 2Al s 3H2SO4 aq Al2 SO43 aq 3H2 g B. A 08838-g sample of an ionic compound containing bromide ions and an unknown metal cation is dissolved in water.
The electrons that are lost in the oxidation reaction are the same electrons that are gained in the 1. Which of the following best describes the redox reaction occurring in the hydrogen fuel cell. 4 What is the reducing agent in this reaction.
2 AI s 3H_2 SO_4 aq rightarrow AI_2 SO_4_3 3H_2 g 2KBr aq Pb NO_3_2 aq rightarrow 2KNO_3 aq PbBr_2 s CaBr_2 aq H_2 SO_4 aq rightarrow CaSO_4 s 2HBr g H aq OH- aq rightarrow H_2 O I CO_32- aq HSO_4- aq rightarrow HCO_3- aq. Hence silver was oxidized and hydrogen was reduced. Zn s -- Zn2 aq 2e-.
2 What is the overall equation for the chemical reaction Zn. In the reaction MgCl2MgCl2 the correct half-reaction for the oxidation that occurs is A. 2Ags H2Sg Ag2Ss H2g represents a redox reaction because the oxidation number of silver increased from zero to 1 while the oxidation number of hydrogen decreased from 1 to zero.
The oxidation half-reaction occurs before the reduction half-reaction. A redox reaction consists of 2 parts oxidation and reduction These two parts can be written up as half-equations where one half-equation shows oxidation and the other shows reduction. Which one of these equations describes a redox reaction.
Chemistry questions and answers. Reactions like double decompositions acid-base neutralization reactions precipitation reactions are non-redox reactions. Tap again to see term.
Which equation is a half reaction that describes the reduction that is taking place. The information below describes a redox reaction.

Solved Which Equation Describes A Redox Reaction 2 Ai S Chegg Com

Solved 11 Which One Of These Equations Describes A Redox Chegg Com

4 Ways To Calculate Molarity Wikihow Molarity Equation Chemistry Notes Calculator

Balancing A Redox Equation In Acidic Solution Worked Example Video Khan Academy
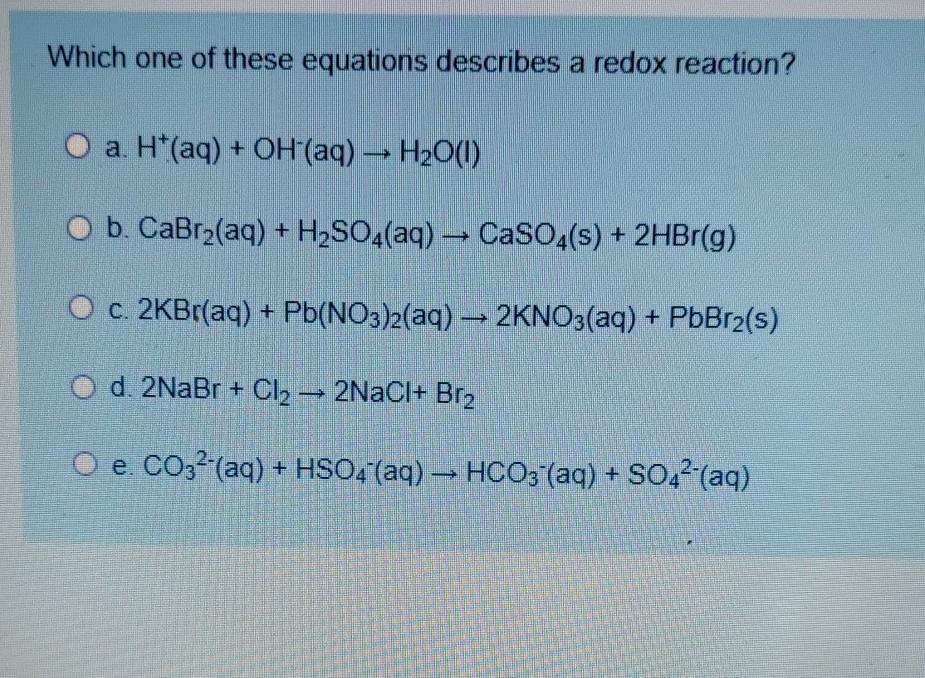
Solved Which One Of These Equations Describes A Redox Chegg Com

Half Reaction Easy Science Redox Reactions 10th Grade Science Easy Science

The Information Below Describes A Redox Reaction What Is The Coefficient Of Silver In The Final Brainly Com

17 Which Ionic Equation Describes A Redox Reaction A Ag Aq Cl Aq Agcl S B 2h Aq Co32 Aq Co2 G H2o L C H Aq Oh Aq H2o L D Zn S Cu2 Aq

Redox Wikipedia The Free Encyclopedia Galvanic Cell Electrochemistry Chemistry

Is Every Reaction Between Two Elements A Redox Reaction Quora

Chemical Reactions Chemical Reaction A Unit Process In Which One Chemical Substance Sequence Is Transfo Chemical Reactions Redox Reactions Chemical Equation
What Is A Redox Reaction Explain With An Example

Which Of The Following Chemical Reactions Is A Redox Reaction Ppt Download

Physical Chemistry Positive Or Negative Anode Cathode In Electrolytic Galvanic Cell Chemistry Stack Exch Electrochemistry Chemistry Classroom Galvanic Cell




Comments
Post a Comment